Since 2021, three expert consensuses on solid tumor MRD testing have been published, with increasingly detailed technical recommendations, reflecting deepening understanding of MRD testing in precision medicine and the gradual establishment of standardized testing criteria in the industry.
"Expert Consensus on Molecular Residual Disease (MRD) in Non-Small Cell Lung Cancer (2021 Edition)" Basic technical requirements for NSCLC MRD testing:
a) The standard is stable detection of ctDNA with an abundance ≥ 0.02%;
b) MRD reports must include cfDNA abundance, ctDNA abundance, and variant allele frequency (VAF) of detected genes.
"Chinese Expert Consensus on Detection and Clinical Application of Molecular Residual Disease (MRD) in Gastric Cancer (2023 Edition)" Technical recommendations for gastric cancer MRD testing:
a) Tumor-informed analysis may better meet clinical needs;
b) ≥ 10 mL of peripheral blood should be drawn each time to improve ctDNA detection sensitivity;
c) Multigene panel testing is recommended;
d) Sequencing depth > 30,000× is required for perioperative gastric cancer patients.
"Consensus on Molecular Residual Disease (MRD) Testing in Solid Tumors (2024 Edition)" Technical recommendations:
a) In future clinical practice, population-based customization, personalized customization, or a combination thereof may be selected after comprehensive evaluation;
b) Tumor-informed analysis strategies are prioritized;
c) Landmark testing (first post-treatment MRD assessment) for solid tumors is typically conducted within 1 month after radical treatment, and dynamic monitoring improves detection performance;
d) MRD testing must undergo thorough performance validation before clinical application, with performance evaluated at both patient and sample levels;
e) Tracking multiple mutations is recommended, with sequencing depth > 30,000× (personalized panels may consider > 100,000×);
f) Quality control (QC) materials containing known positive/negative mutations should be included to monitor assay stability and accuracy;
g) Reports must include testing scope, methodology, QC results, MRD positivity/negativity criteria, limit of detection, and limitations.
l Zero-Cost Customization of Personalized MRD Panels
As a reagent supplier specializing in targeted capture and MRD solutions, iGeneTech's free custom MRD Panel service has been adopted by multiple clinical institutions and laboratories. Based on a tumor-informed strategy, each panel allows free synthesis of up to 150 probes. Compared to conventional column-based single-probe synthesis, this approach minimizes costs while rapidly meeting customization needs for large sample volumes.
l MRD Probe Express Delivery in 5 Working Days
This rapid delivery capability relies on iGeneTech's proprietary high-throughput nucleic acid synthesizer, enabling precision control throughout the entire process. Upon receiving user-submitted MRD loci, the system automatically designs probes, supplements mutant-type probes, and completes design-to-synthesis order placement, ensuring delivery within 5 working days.
l Full-Process Reagent Solutions
After years of independent R&D on reagents and application expansion across diverse scenarios, iGeneTech has developed a complete MRD solution covering sample extraction, library preparation, capture, and bioinformatics analysis. Each component is compatible with automated equipment for stable and efficient operation.
Practical Test Data of iGeneTech MRD Panel
Probe Storage Stability Test
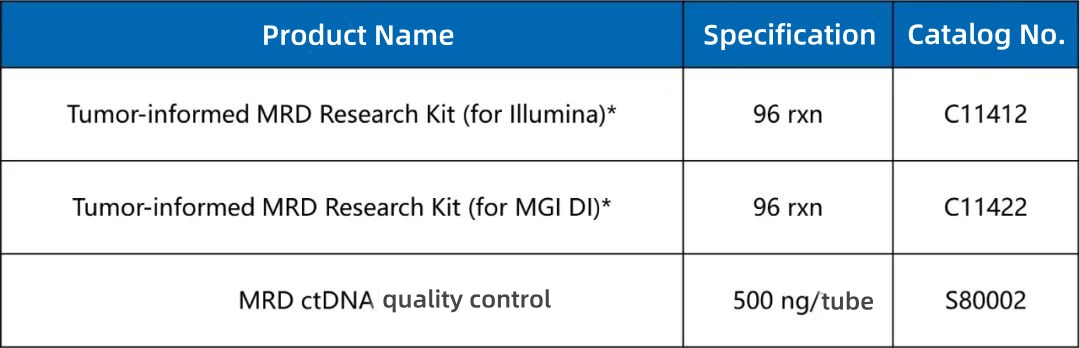
After storing MRD Panel probes at room temperature for 3 and 7 days (control probes stored at -20°C), capture tests were conducted. Results show:
· No significant reduction in capture performance after 3–7 days at room temperature
· On-target rate > 75%
· 100% of regions achieved depths exceeding 50% of the average depth
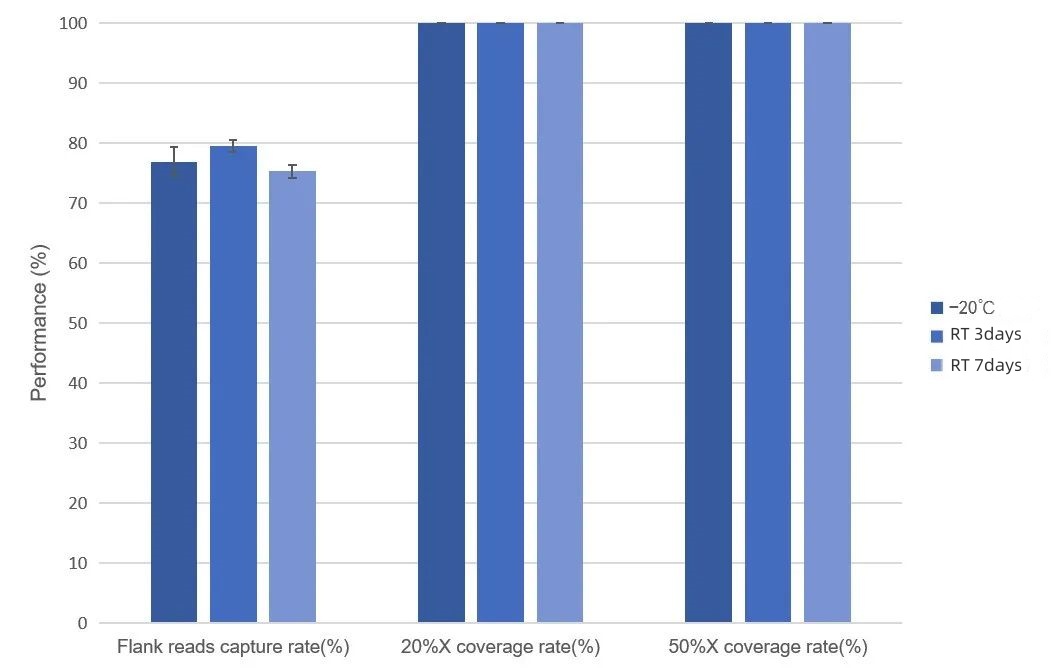
Figure 1. Data Performance of the Storage Stability of MRD Panel Probes. The MRD Panel with 34 loci was used for the test. The input amount of the single hybridization library was 750 ng, and the sequencing was carried out on the NovaSeq 6,000 platform with PE150 mode.
Probe Stability Test under Repeated Freezing and Thawing
The probes of the MRD Panel were subjected to capture tests after being repeatedly frozen and thawed 10 times and 20 times respectively. The control probes were stored at -20 degrees Celsius. The test data shows that there is no significant decrease in the capture performance of the MRD Panel after being repeatedly frozen and thawed 10 times and 20 times. The on-target rate is > 58%, and the depth of 100% of the regions exceeds 50% of the average depth.
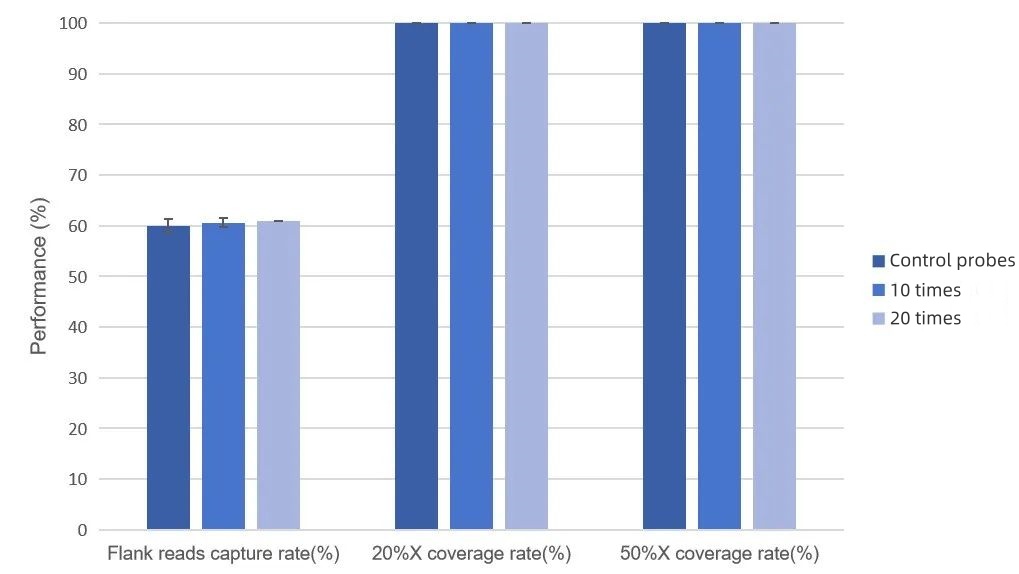
Figure 2. Data Performance of the Freezing and Thawing Stability of MRD Panel Probes. The MRD Panel with 25 loci was used for the test. The input amount of the single hybridization library was 750 ng, and the sequencing was performed on the NovaSeq 6,000 platform with the PE150 mode.
Capture Performance of MRD Panels of Different Sizes
Based on iGeneTech's overall MRD Panel solution, the capture performance tests were carried out on 33 different MRD Panels (containing 15 to 150 loci) synthesized for 33 patients in the real world according to the tumor-informed strategy. All the tested MRD Panels demonstrated excellent capture performance: the on-target rate was > 55%, the coverage was 100%, the depth of > 97% of the regions exceeded 20% of the average depth, and the depth of > 91% of the regions exceeded 50% of the average depth.
Figure 3. Data Performance of the Capture Tests of Different MRD Panels. The capture tests were conducted using 33 MRD Panels. The input amount of the single hybridization library was 750 ng, and the sequencing was carried out on the NovaSeq 6,000 platform with PE150 mode.
Results of Technical Performance Test
An MRD panel with 37 loci was used to detect ctDNA standard samples with different mutation frequencies. The detection sensitivity of both 0.1% and 0.01% ctDNA standard samples at the sample level reached 100%.
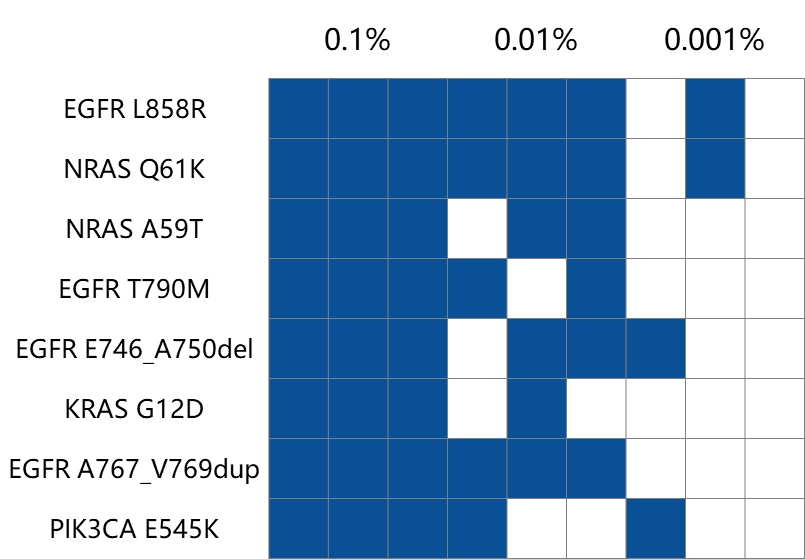
Horizon ctDNA standard samples (HD780, containing 8 types of mutations) with mutation frequencies of 0.1%, 0.01%, and 0.001% were used for library preparation with an input amount of 50 ng (3 technical replicates). The MRD Panel with 37 loci was used for capture. The sequencing data was analyzed using the UMI workflow built by iGeneTech. A sample was defined as positive for detection if ≥ 2 types of mutations were detected at the sample level.
Order Information
Based on Tumor-informed assays, iGeneTech has launched a complete set of MRD hybridization capture reagents, which include hybridization capture reagents, blocking oligo, and capture magnetic beads. Combined with the free custom MRD Panel, it forms an MRD detection solution with high cost-effectiveness and high stability, promoting the popularization of the clinical application of MRD detection.