The NCCN Clinical Practice Guidelines Update (2025 V1) incorporates important MRD - related information: In the field of B - cell lymphoma, for patients with diffuse large B - cell lymphoma (DLBCL) who have a positive positron emission tomography (PET) scan at the end of first - line treatment, circulating tumor DNA (ctDNA) testing is newly added for MRD evaluation [1 - 2]. This update provides clinicians with a more accurate and scientific basis for MRD evaluation in the diagnosis and treatment decisions of related cancers.
Signatera continues to dominate the MRD market with remarkable achievements: As a benchmark product in the field of MRD testing, Signatera has been maintaining its leading position in the market performance. Throughout 2024, the number of its MRD tests soared to 499,000 cases. Compared with the 303,000 cases in 2023, the growth rate was as high as 64.7%. Among the tumor testing items, the proportion of the sample quantity for MRD testing has been rising all the way and currently has reached an impressive 96% [3]. With the in-depth progress of numerous clinical trials, the effectiveness and safety of Signatera have been fully verified. At the same time, the coverage of medical insurance is also expanding continuously, which greatly improves the accessibility of this test for patients and brings hope of precise diagnosis and treatment to more cancer patients.
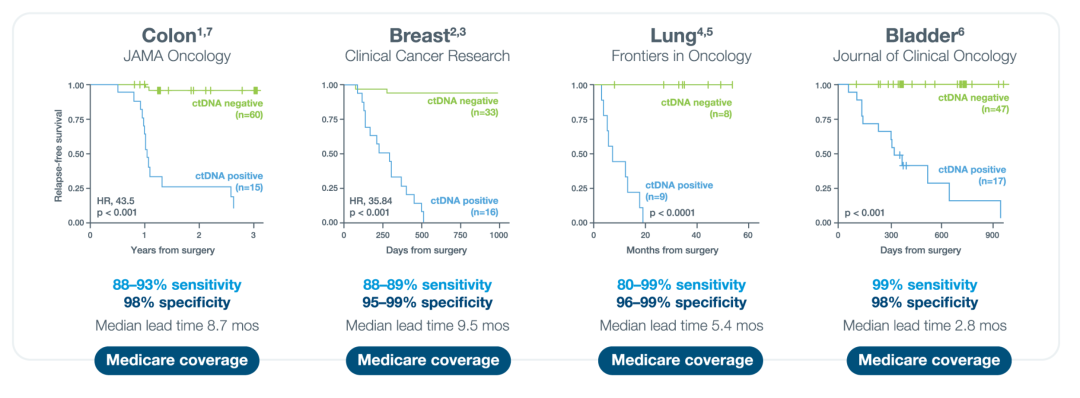 The research achievements of Professor Wu Yilong's team on minimal residual disease (MRD) in early-stage non-small cell lung cancer (NSCLC) were published: In January 2025, the latest follow-up results of the prospective cohort study on MRD in early-stage NSCLC conducted by Professor Wu Yilong's team were successfully published in the international top journal Clinical Cancer Research (Impact Factor: 10.4) [4]. This study deeply analyzed the predictive value of MRD in the early-stage NSCLC cohort, clarified its lead time for predicting recurrence in advance and its dynamic changes. It provided key evidence for the clinical application of MRD in early-stage NSCLC, further consolidated the important position of MRD in this field, and is expected to promote the innovation of the diagnosis and treatment mode for early-stage NSCLC.
The research achievements of Professor Wu Yilong's team on minimal residual disease (MRD) in early-stage non-small cell lung cancer (NSCLC) were published: In January 2025, the latest follow-up results of the prospective cohort study on MRD in early-stage NSCLC conducted by Professor Wu Yilong's team were successfully published in the international top journal Clinical Cancer Research (Impact Factor: 10.4) [4]. This study deeply analyzed the predictive value of MRD in the early-stage NSCLC cohort, clarified its lead time for predicting recurrence in advance and its dynamic changes. It provided key evidence for the clinical application of MRD in early-stage NSCLC, further consolidated the important position of MRD in this field, and is expected to promote the innovation of the diagnosis and treatment mode for early-stage NSCLC.

Important research achievements released by the Cancer Center of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology: In February 2025, the results of a multicenter, randomized, open-label Phase III clinical trial carried out by the Cancer Center of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology were published in the authoritative journal "Cancer letters" (IF = 9.1) [5]. Through longitudinal MRD monitoring, this study evaluated the feasibility of combining camrelizumab and chemotherapy after short-course radiotherapy as neoadjuvant therapy (NAT) for locally advanced rectal cancer (LARC), aiming to explore the value of minimal residual disease (MRD) based on circulating tumor DNA (ctDNA) in comparing the efficacy of short-course CRT and long-course CRT. This research provides new ideas and evidence for the selection of clinical treatment regimens for LARC, and helps to promote the development of LARC treatment towards a more precise and efficient direction.
Growth in Customization Quantity: Compared with Q4 of 2024, the quarter-on-quarter growth rate of the customized MRD panel quantity in Q1 of 2025 reached as high as 80%.
Extensive Collaboration: The company has cooperated with more than 20 leading central laboratories, covering over a hundred hospitals.
Fast Delivery Cycle: The fastest delivery cycle can be achieved within 4 natural days.
The rapid growth of MRD products largely benefits from the support of the end-to-end online design website. This allows customers to log in to their accounts at any time for online design, self-service order placement, logistics progress inquiry, and order management. Combined with the company's internal automated information management system, it enables an extremely short delivery cycle.
Three Unique Highlights of the Customized MRD Panel
Overlapping Probe Design Scheme: It supports the online selection of overlapping probe designs with a multiplier of 3× and higher. This is to ensure stable detection in difficult-to-capture regions such as those with high GC content and introns.
For InDel (insertion-deletion) and even fusion mutations during monitoring, it supports customers in choosing the mutant probe design scheme. By targeting and capturing with probe sequences that are exactly the same as the mutant sequences, the ability to detect mutations is significantly enhanced.
For the fixed hotspots panel + individualized customization solution, the information of the fixed panel can be deployed online. Each time you submit individualized loci, you just need to click the "Merge Fixed Panel" option to merge and synthesize the probes and integrate the regions required for analysis.
iGeneTech's Tumor-informed MRD Research
Based on Tumor-informed assays, combined with the independently developed hybridization capture system and high-throughput MRD Panel synthesis platform, a complete set of MRD hybridization capture reagents including hybridization capture reagents, blocking sequences and capture magnetic beads is launched. Combined with the free customized MRD Panel, it forms a cost-effective and highly stable MRD detection solution, promoting the popularization of the clinical application of MRD detection.
Features
· Stable and Efficient Process with Supporting Reagents: A complete set of reagents can be used for both initial screening and monitoring, and the reagents in each link can stably perform their functions, reducing process obstacles. This enables patients to quickly obtain accurate test results and accelerate the diagnosis and treatment process.
· Highly Compatible Hybridization Capture Reagents: It can seamlessly interface with all types of probe products (A*, I*, R*). Whether it is a complex multi-target detection or an urgent need for rapid hybridization, it can be perfectly adapted. It provides diversified detection options for different patient groups, expands the detection coverage, and benefits more patients.
· High-throughput MRD Panel Customization: A zero-cost individualized MRD customization solution for patients reduces the cost of medical testing and thus alleviates the economic burden on patients.
· Rich Individualized Monitoring Sites in the Individualized MRD Panel: The number of individualized monitoring sites can be increased to 150, significantly improving the detection sensitivity. It can accurately capture minimal residual lesions, helping doctors detect disease changes earlier and formulate more timely and precise treatment plans for patients.
Performance
The Capture Performance of MRD Panels of Different Sizes
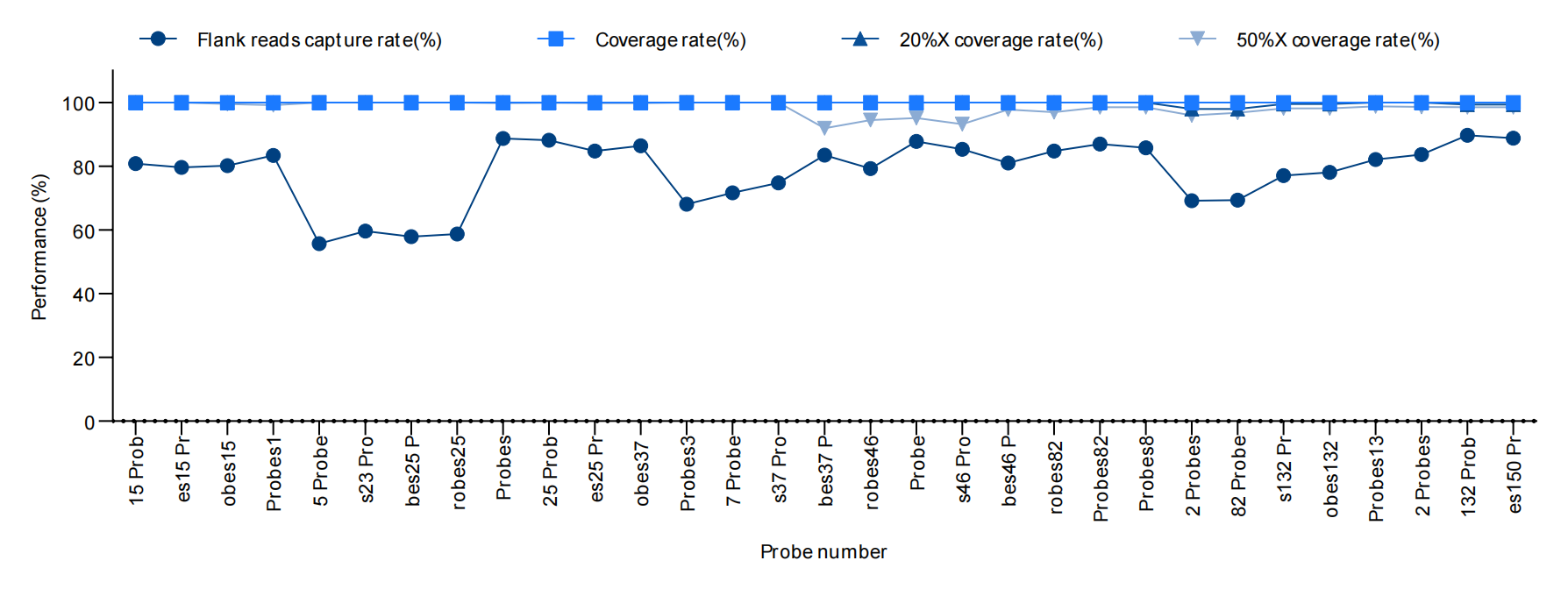
Figure 1. The test statistical results of MRD Panels with different numbers of probes are shown. Based on iGeneTech's overall MRD Panel solution, the capture performance tests were conducted on 33 synthesized different MRD Panels (including 15 to 150 loci). The individualized customized Panels for different monitoring loci obtained from the initial screening can all be formed at one time and can be used stably and efficiently. Even for a small panel with only 15 probes, combined with iGeneTech's capture system, the capture efficiency can still be maintained above 60%.
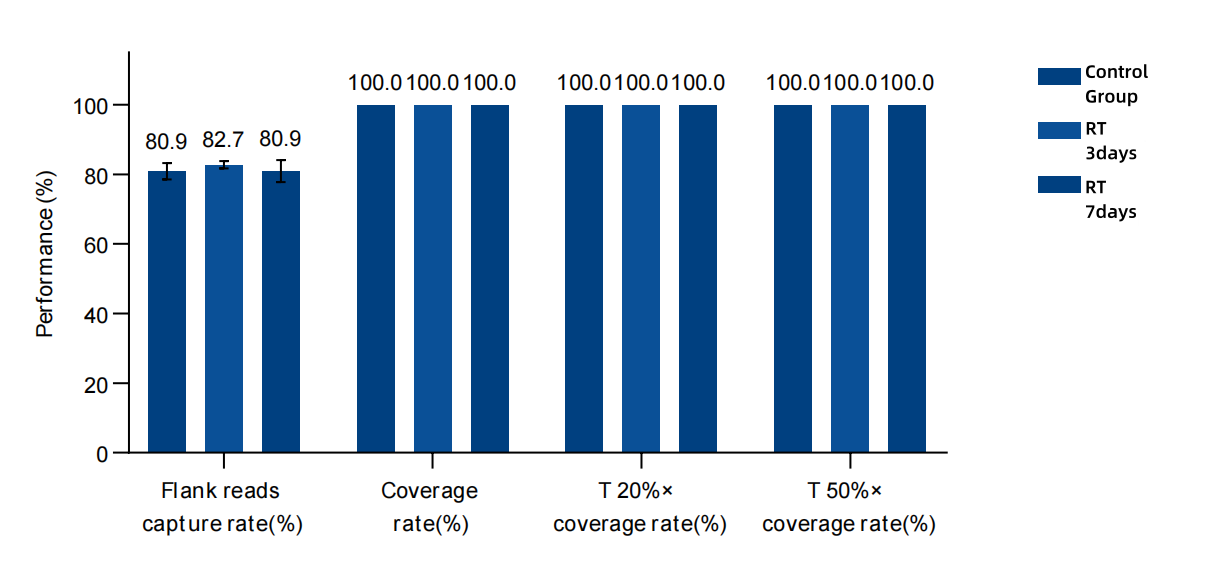
Figure 2. The data performance of the storage stability of MRD Panel probes. An MRD Panel with 34 loci was used for the test. The input amount of the single-hybrid library was 750 ng, and the sequencing was carried out on the NovaSeq 6,000 platform with PE150. Through the accelerated stability experiment, two storage conditions of being stored at -20°C for 1 year and 3 years were simulated. The results show that the customized Panel can be stably stored, ensuring that the effect can still be guaranteed during subsequent continuous monitoring and use.
Stability Test of Probes under Repeated Freezing and Thawing
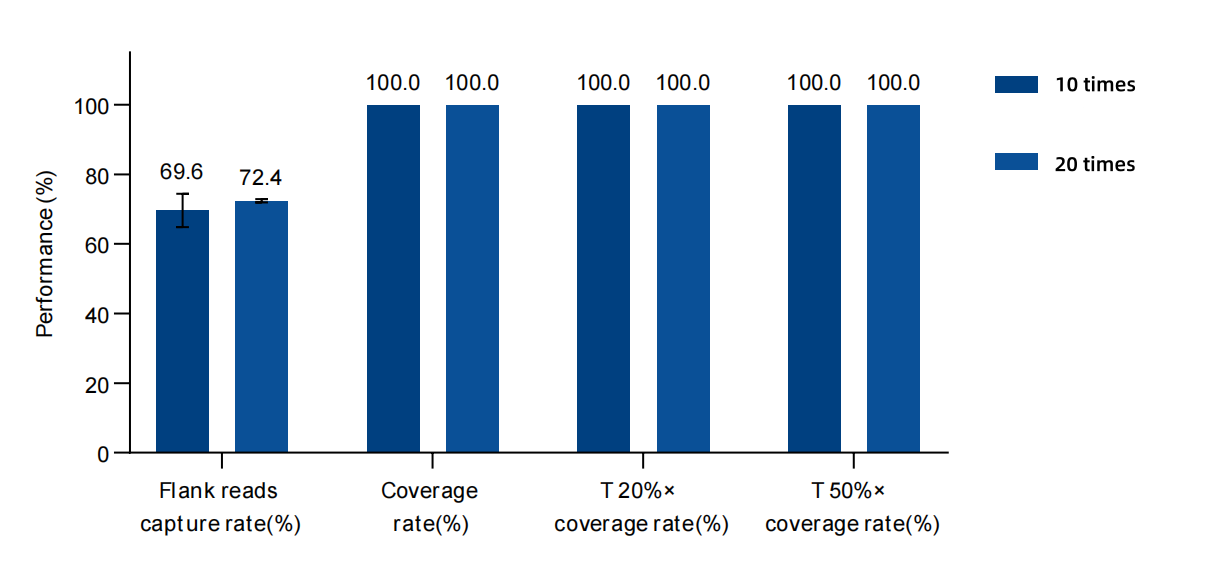
Figure 3. The data performance of the freezing-thawing stability of MRD Panel probes. An MRD Panel with 25 loci was used for the test. The input amount of the single-hybrid library was 750 ng, and the sequencing was carried out on the NovaSeq 6,000 platform with PE150. After the probes of the MRD Panel were repeatedly frozen and thawed 10 times and 20 times, capture tests were conducted. The control probes were stored at -20°C. The test data shows that the capture performance of the MRD Panel remains stable after being repeatedly frozen and thawed 10 times and 20 times.
Low-frequency Variant Detection Ability
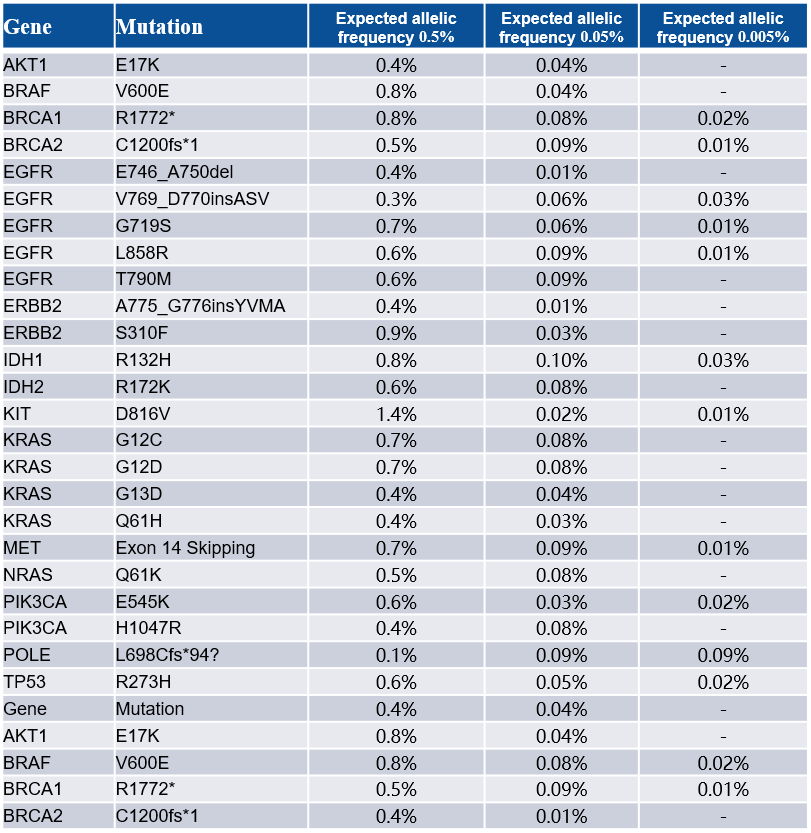
Table 1. Tests of Different Frequency Gradients of cfDNA Positive Reference Samples
Note: Samples of cfDNA at four gradients of 0.5%, 0.05%, 0.005%, and 0% from the MRD reference materials of Shuimu Jiheng were used for the test. The input amount was 50 ng, with a Panel of 24 high-throughput synthesized probes. The raw bases were 1 Gb, and the depth before deduplication was approximately 180,000×.
References
[1]. NCCN Guidelines updated to include ctDNA-MRD testing recommendation for B-cell lymphoma. Foresight Diagnostics. January 10, 2025. Accessed January 10, 2025.
[2]. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) B-Cell Lymphomas Version 1.2025. December 20, 2024. Accessed January 10, 2025.
[3]. https://d18rn0p25nwr6d.cloudfront.net/CIK-0001604821/54a5b505-aa6f-4124-b301-caf3cff3d759.pdf
[4]. Follow-Up Analysis Enhances Understanding of Molecular Residual Disease in Localized Non-Small Cell Lung Cancer. Clin Cancer Res. 2025 Jan 24. doi: 10.1158/1078-0432.CCR-24-2909. Epub ahead of print. PMID: 39853318.
[5]. Longitudinal circulating tumor DNA monitoring in predicting response to short-course neoadjuvant radiotherapy in locally advanced rectal cancer: Data from a phase III clinical trial (UNION).. JCO 42, LBA3606-LBA3606(2024).