Overview
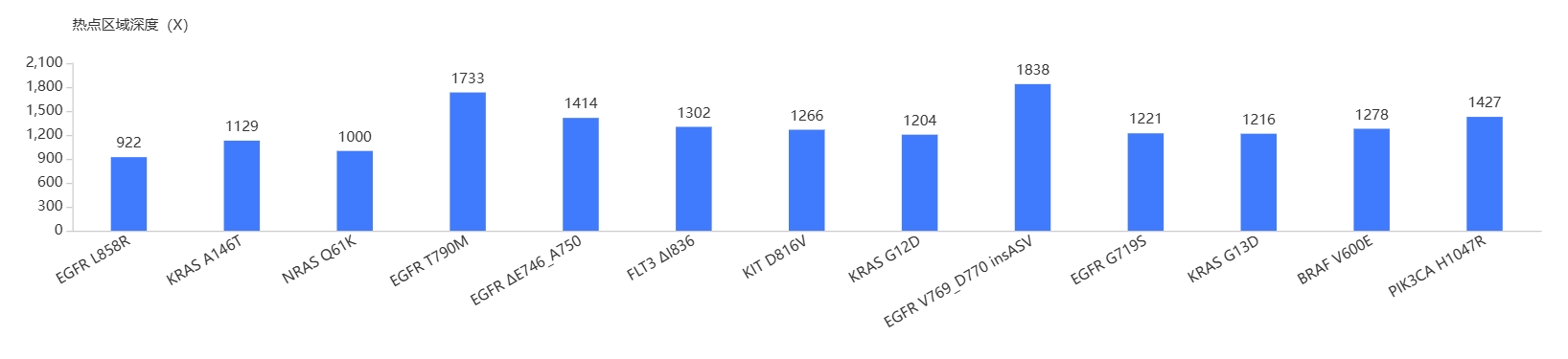
AIExome® Human Exome Panel V3 – Tumor is a version of AIExome® V3 specifically designed to detect tumor related mutations. In addition to the superior coverage of CDS region as AIExome® V3, AIExome® V3 – Tumor also covers important fusions, MSIs, HLA regions and tumor related genes.

 CN
CN