On July 22, 2021, the SARS-CoV-2 Clinical Amplicon Sequencing Kit independently developed by iGeneTech passed the EU IVDD CE certification and obtained the in vitro diagnostic reagent registration license issued by the Dutch CIBG authority: NL-CA002-2021-60454, and obtained the EU access qualification. As a result, iGeneTech can provide high-quality monitoring and traceability services for new coronavirus mutations in more countries around the world, and will continue to give full play to the advantages of domestic reagents and contribute to the global fight against the epidemic.

Wide range of application: suitable for throat swabs, nasal swabs, deep cough sputum, alveolar lavage fluid, environmental samples and other types of samples;
Comprehensive detection: the detection coverage is >99.8%, and the virus sequence variation analysis can be carried out, covering all mutant strain species, including: α strain (B.1.1.7), β strain (B.1.351), γ strain (P.1), δ strain (B.1.617.2);
Simple experiments: the multiplex PCR technology platform has simple experiments, and only 2 rounds of PCR can complete the genome amplification and library construction of viruses;
Applicable platform: PE150 sequencing mode, suitable for illumina and MGI sequencing platforms;
Cost-effective: Relying on the independent intellectual property technology platform, the detection cost is greatly reduced.
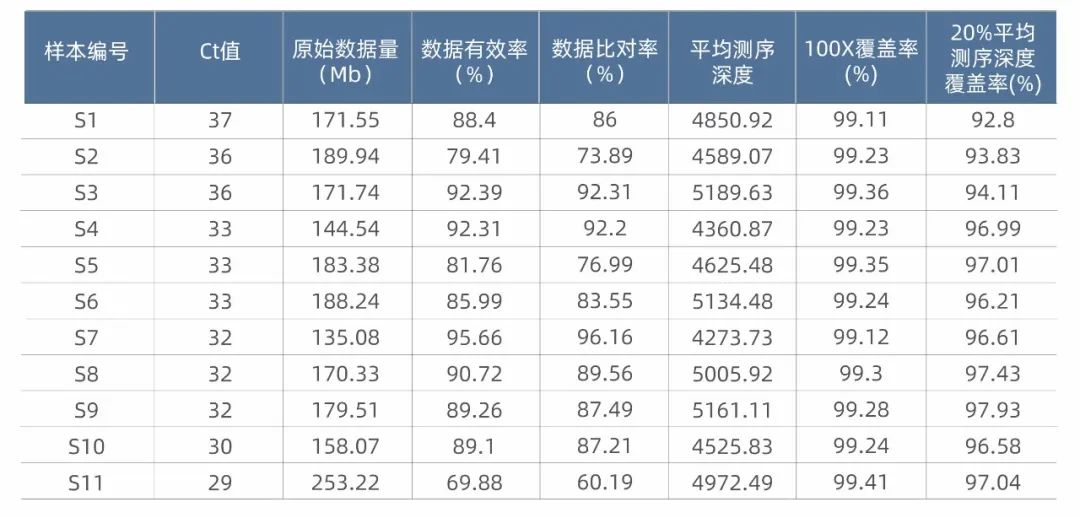
The test statistics of the SARS-CoV-2 Clinical Amplicon Sequencing Kit show that the detection sensitivity and coverage of this product are excellent. For samples with a Ct value below 38, 100X coverage can reach more than 99%, and the 20% average sequencing depth coverage is excellent.