The MicroArray/Sequencing Quality Control (MAQC) association led by the US FDA has carried out the Analytical Performance Evaluation and Verification Project (SEQC2) based on next-generation sequencing technology worldwide. In this project, MAQC Association has carried out cross-platform analysis performance verification of multiple kit manufacturers and laboratories in multiple subdivision detection fields such as tumor targeted capture sequencing, whole exome sequencing and methylation sequencing, with the aim of establishing industry standards and providing technical guidelines through performance comparison analysis, and recommending reliable next-generation sequencing reagent suppliers for global scientific research and clinical researchers.
In this SEQC2 project, eight sequencing reagent manufacturers, including iGeneTech, Agilent, Roche, IDT, Illumina, and Qiagen, participated in the analysis performance verification of pan-tumor targeted capture sequencing kits, and the relevant evaluation results were published in Genome Biology (IF=8.2021): Cross-oncopanel study reveals high sensitivity and in April 4 accuracy with overall analytical performance depending on genomic regions. The kit that iGeneTech participated in the project is the NCC Tumor Diagnostic Kit (AIOnco-seq), which covers 10 gene whole exon regions proposed by the National Cancer Center in Japan, which can provide a reference for tumor diagnosis and hereditary tumor risk prediction, and realize precision medicine. The test evaluation results show that the NCC Tumor Diagnostic Kit (AIOnco-seq) is at the leading level in terms of detection accuracy, sensitivity and reproducibility.
▌Sample type
sample A (genomic DNA from a mixture of 10 cancer cells from Agilent); sample B (healthy human cell line for men); sample C (sample A and sample B are mixed in equal proportions); Sample D (sample B mixed with 5% Thermo Fisher Scientific AcroMetrix tumor hotspot control).
▌Scale of experiment
28 independent laboratories with a total of 430 libraries (each panel is experimented in at least 3 laboratories).
▌Evaluation indicators
Sensitivity, false positive rate, reproducibility.
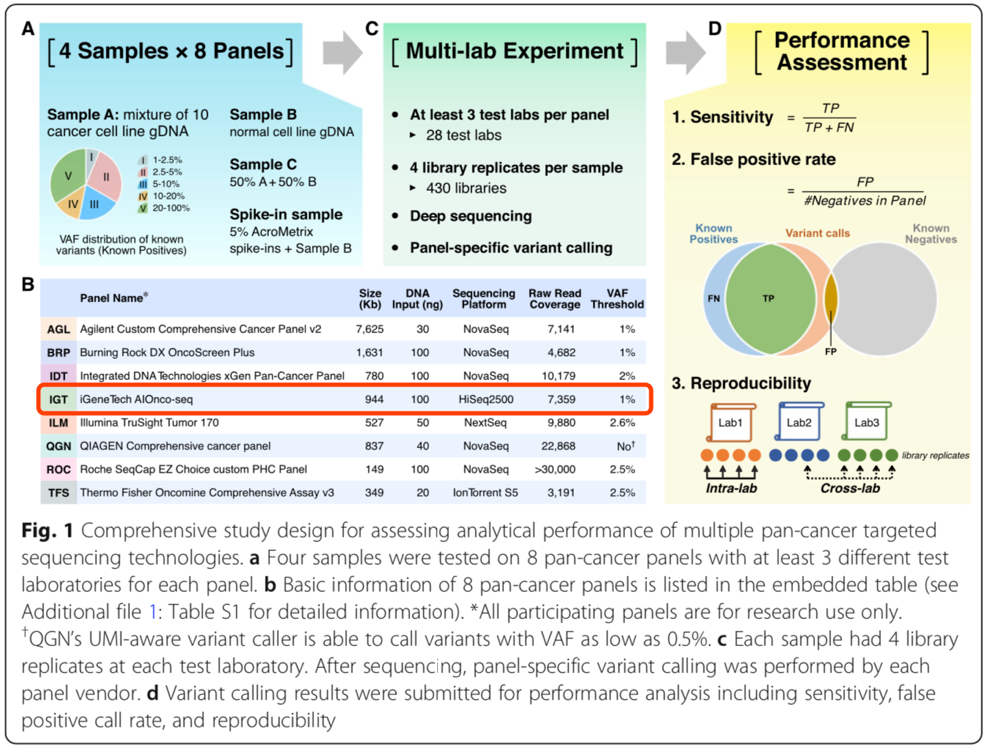
▌Sensitivity test results
In the range of high-frequency mutations (10% <VAF<20%), all panels performed with excellent sensitivity. However, in the range of low-frequency mutations (1% <VAF<2.5%), the sensitivity performance of different panels varies. Among them, the AIOnco-seq kit (IGT) has a sensitivity >of 1% when the VAF is 2-5.97%; At VAF > 2.5%, the sensitivity is as high as 100% (Figure 2A), which is excellent.
▌False positive test results
The analysis results showed that when VAF was elevated, the false positive rate (FP) was significantly reduced. As shown in Figure 2B, when VAF >5%, the false positive rate of all panels is close to 1/Mb; At a VAF > 10%, no false-positive sites were detected.
▌Repeatability test results
Cross-platform and same-platform replication results showed that AIOnco-seq products were reproducible up to 100%, the best performance of all products (Figure 2C).
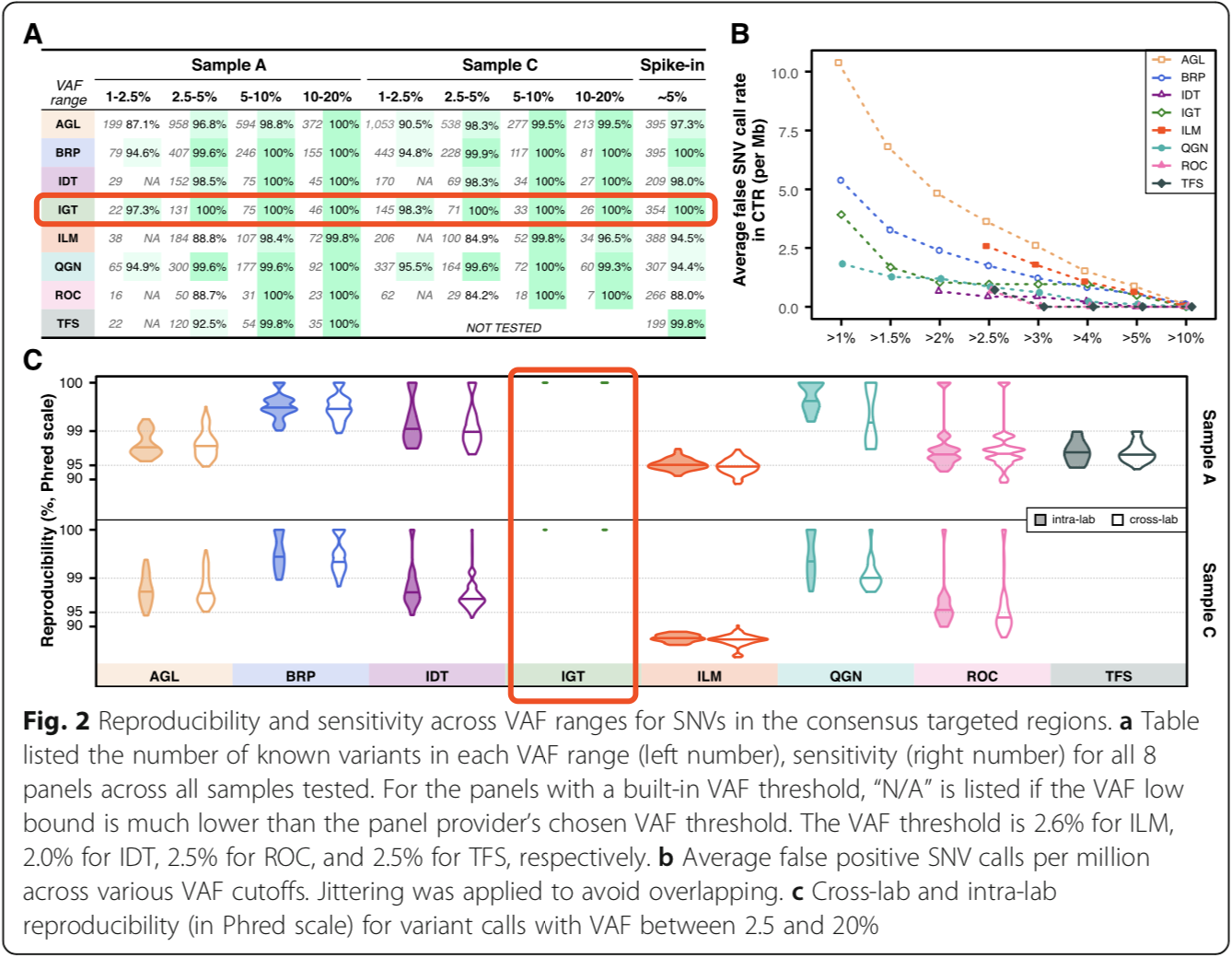
▌Influence of mutation type on detection sensitivity
Different types of variants can lead to differences in detection sensitivity, but AIOnco-seq (IGT) products have 100% sensitivity in both SNV and non-SNV (small indels and MNV) detection (Figure 3A). Comparing the sensitivity differences caused by VAF cutoff in different sample types, the results show that when VAF cutoff decreases, the sensitivity increases accordingly, and the trend is consistent in the detection of each sample type, as shown in Figure 3B, when VAF >2%, the sensitivity of AIOnco-seq products >99%. Combined with the overall evaluation of sensitivity and reproducibility, the AIOnco-seq kit has the best detection accuracy.
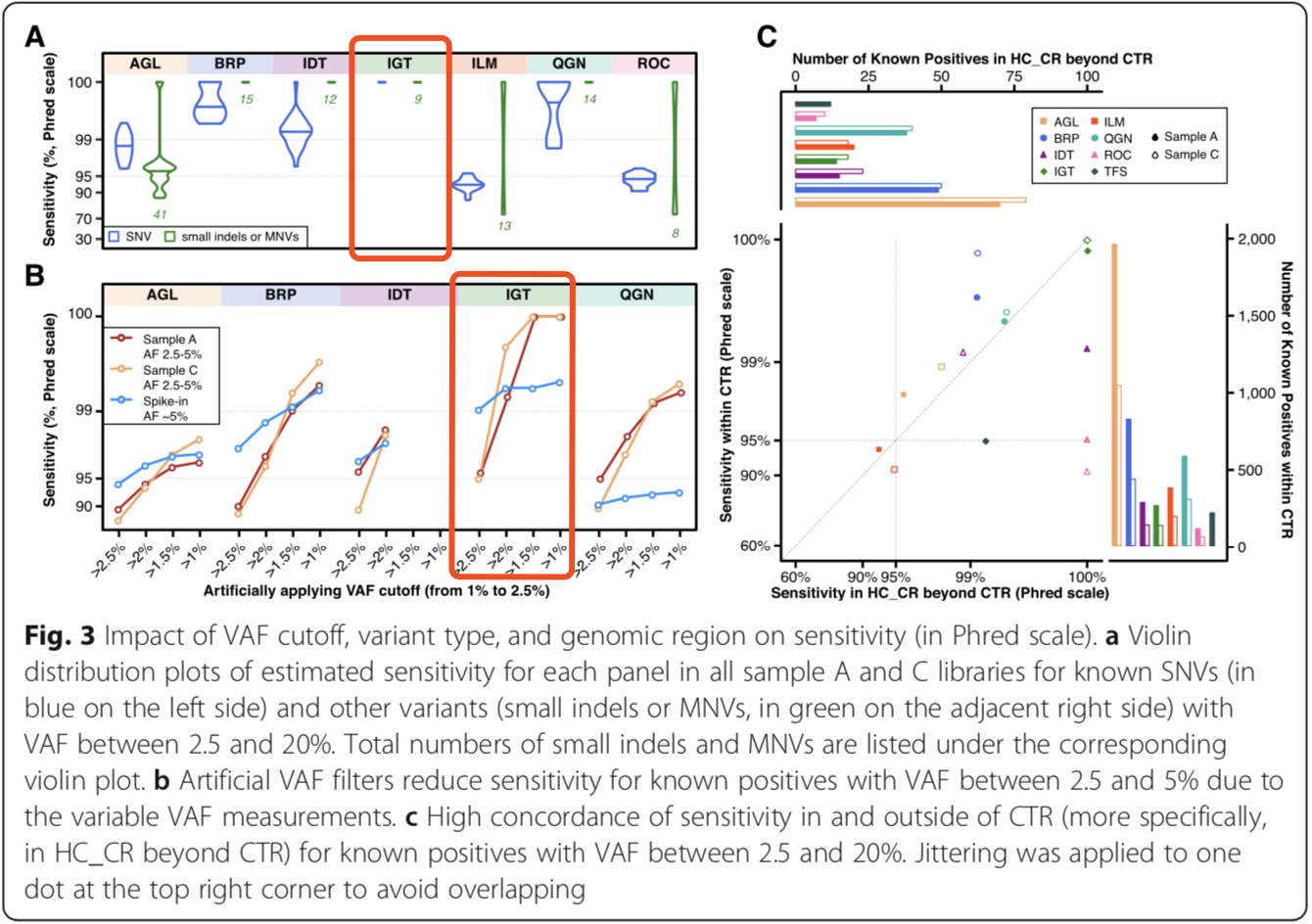
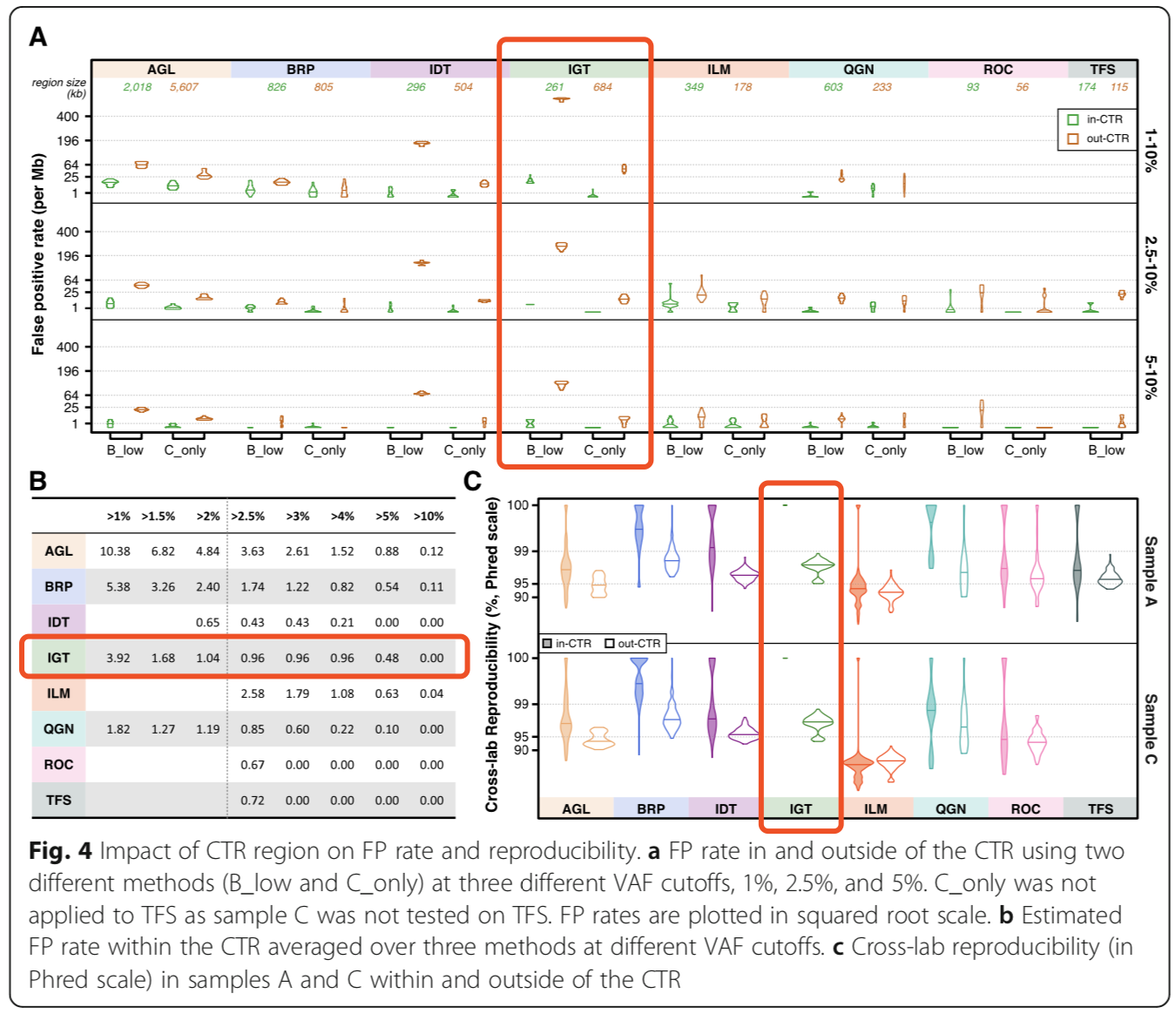
Within the region of interest (CTR), all panels showed lower false-positive rates and higher reproducibility in medium-high frequency variation (2.5%<VAF<10%), but increased false-positive rates outside the target region with reduced reproducibility (Figure 4). Figure 4C shows up to 100% reproducibility of the AIOnco-seq kit in the CTR region.
Based on the differential evaluation data between all platforms, AIOnco-seq has excellent performance, with high sensitivity, low false positive rate and high reproducibility, which can ensure the accuracy and stability of clinical tumor gene mutation detection. In the future, we will uphold our original intention, continue the industrialization and development of gene capture technology, and launch more efficient, stable and reliable tumor diagnosis products.