Why conduct MRD testing?
During the treatment process of tumors, it is crucial for tumor patients to undergo regular reexaminations and follow-ups. Early detection and early treatment can help seize the best treatment opportunity, control the disease condition in a timely manner, and maximize the benefits for patients. Routine reexamination items include traditional imaging methods (such as CT, PET-CT), biochemical tests, tumor markers, etc. However, biochemical indicators have poor sensitivity and specificity; when lesions are observed through imaging methods, it indicates that the tumor has already recurred, and it is easy to miss the best treatment opportunity. Therefore, the monitoring after tumor surgery requires a detection method that is both sensitive and specific, so as to detect earlier whether the tumor has recurred, and the detection results must be accurate and reliable. MRD testing based on ctDNA can meet the above two requirements. A number of studies have already proven that ctDNA MRD testing can predict recurrence on average 3 to 10 months or more earlier than clinical imaging [1-3].
How to Improve the Accuracy of MRD Testing
In recent years, numerous clinical studies have shown that MRD testing plays an important role in the post-surgery or post-treatment management of cancers such as lung cancer, colorectal cancer, gastric cancer, and breast cancer, the screening of patients for immunotherapy, the selection of treatment methods, and the decision-making for drug holidays [4-7]. However, there are still many difficulties in the clinical practical application and promotion of MRD. Whether patients are willing to accept subsequent blood collection, the accuracy of MRD testing, the testing cost, etc., are all problems faced in clinical practice. Among them, the most primary issue is how to ensure that minimal residual disease (MRD) can be accurately detected.
First, in terms of the selection of the MRD testing strategy, in order to ensure the accuracy of the test results, the Tumor-informed testing strategy is mostly adopted. Each patient is assigned specific mutation sites, and dynamic monitoring is carried out through ctDNA molecules in peripheral blood during subsequent reexaminations and follow-ups. However, the content of ctDNA in peripheral blood is generally low (less than 1%). And the MRD testing based on NGS mentioned in the "Expert Consensus on Molecular Residual Disease in Lung Cancer (2021 Edition)" requires the stable detection of ctDNA molecules with an abundance of ≥ 0.02%, which poses higher requirements for the testing protocol and testing technology. In order to improve the sensitivity of the test, most current testing technologies address the issue from two aspects:
1. Draw more blood and extract cfDNA from a large volume of plasma for testing
The "Expert Consensus on Molecular Residual Disease in Lung Cancer (2021 Edition)" states that the limit of the molecular signal detectable in 10 mL of plasma is 0.02%. However, in actual clinical testing, the amount of plasma cfDNA is limited. For a 10 mL blood collection tube, the actually separated plasma is only about 4 mL [8]. Therefore, most current MRD testing products require collecting at least 20 mL of whole blood from patients to ensure the sensitivity of subsequent MRD testing. Cancer-related anemia (CRA) is one of the common concomitant diseases of malignant tumors, with a prevalence rate of 30% to 90% [9]. For the physical and mental state of anemic patients, drawing 20 mL of whole blood is quite a burden. Coupled with the blood volume drawn for other blood tests during the reexamination process, this burden will inevitably be further aggravated.
2. Ultra-high sequencing depth
The sequencing depth of NGS (next-generation sequencing) significantly impacts the sensitivity of detection in solid tumor MRD testing [10]. Sequencing depth can be understood as the number of times you "grab balls" from a bucket, where ctDNA is like one or a few small balls. Grabbing 10,000 times gives a higher chance of capturing the ctDNA ball(s) than grabbing 100 times, so increasing the sequencing depth can improve detection sensitivity. However, in practice, low-abundance ctDNA may be lost during sample extraction, quality control, and library preparation. The extracted cfDNA contains both mutated ctDNA molecules (red balls) and unmutated cfDNA molecules (white balls). The process of random sampling in experiments is akin to scooping a small bucket of balls from a large container—when ctDNA accounts for a very low proportion, it may be lost during random sampling. Additionally, in NGS library preparation, only molecules effectively ligated with sequencing adapters will finally appear in the sequencing data for mutation analysis. Therefore, when the number of MRD monitoring sites is small and the blood draw volume is limited, random sampling and library preparation can both lead to ctDNA loss, making it impossible for ultra-high-depth sequencing to achieve accurate detection.
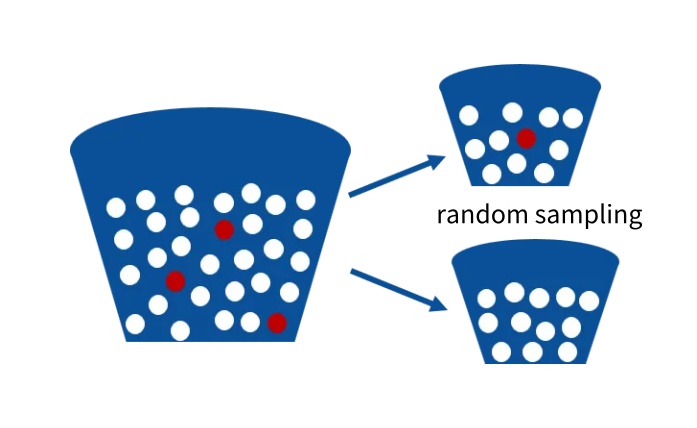
How to Ensure MRD Testing Accuracy While Minimizing Blood Draw?
Even with low blood collection volume and low input amounts, increasing the number of monitoring sites can still enable high-sensitivity MRD testing. This approach balances the physical and psychological well-being of patients while ensuring the sensitivity of ctDNA detection.
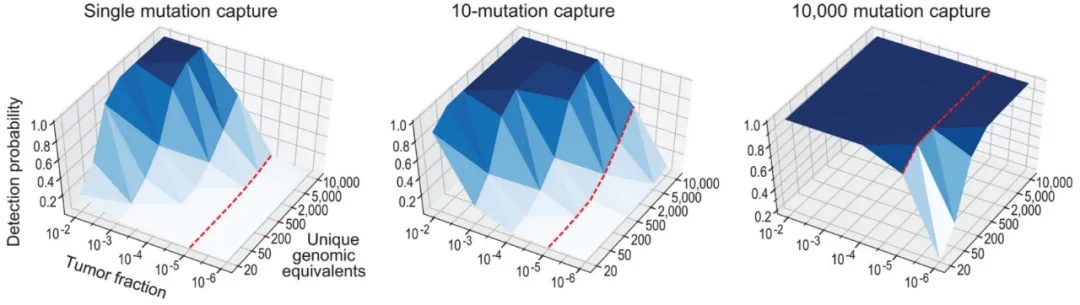
The randomness of plasma sampling also poses a challenge for detecting low-abundance ctDNA, but increasing the number of detection sites can effectively overcome the limitations of low starting sample volume and sampling randomness. As shown in the figure below, when the number of single-nucleotide variants (SNVs) to be detected is 1, the detection probability decreases rapidly as the tumor fraction (TFs, tumor cell content) decreases; when the number of SNVs is 10 and TFs is 10⁻⁴, the detection probability exceeds 80% among 5,000 copies [11].
In addition, Ymke Van Der Pol and others used binomial simulation to calculate the probability of detecting at least one ctDNA molecule in 1 mL of plasma cfDNA (10 ng, 3,000 copies, with 1,500 copies remaining after cfDNA loss). The results showed that increasing the number of detected mutations can theoretically improve the detection of low-abundance ctDNA molecules [12].
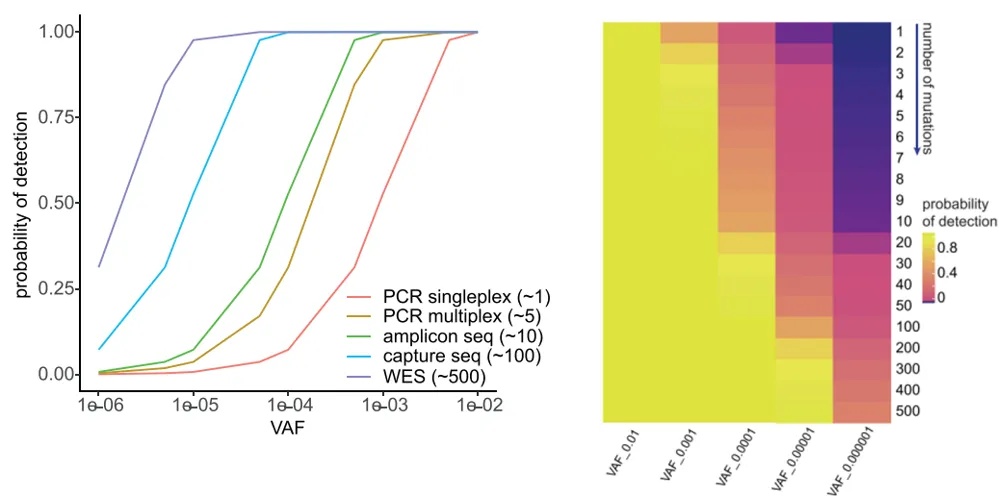
In practice, achieving the theoretically estimated 50% loss for 10 ng (3,000 copies) of cfDNA places high demands on sample and data utilization in subsequent experimental steps such as sample extraction, library preparation, and target capture.
For the extraction step, to efficiently extract cfDNA from a small volume of blood samples, iGeneTech has developed an Enhanced Magnetic Bead-based Large-Volume Cell-Free DNA Extraction Kit, which can isolate and purify high-quality cell-free nucleic acids from a single 1 mL to 4 mL volume of plasma, serum, or other samples, reducing extraction time and manual operation steps.
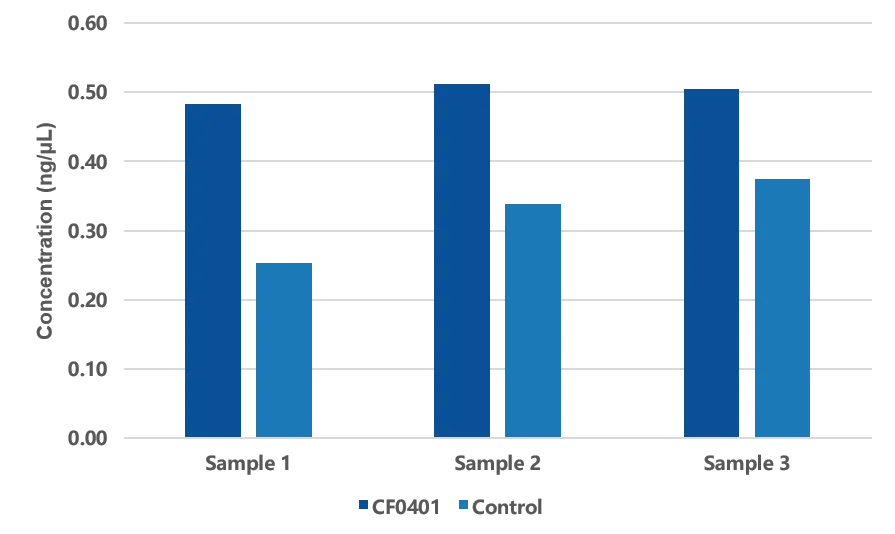
Figure 1. Average concentration of three types of animal plasma model samples after extraction.
For the library preparation step, iGeneTech uses its self-developed IGT® Fast Library Prep Kit v2.0, which can achieve a molecular utilization rate of over 50%, fully ensuring the detection of low-abundance ctDNA even with low input volumes.
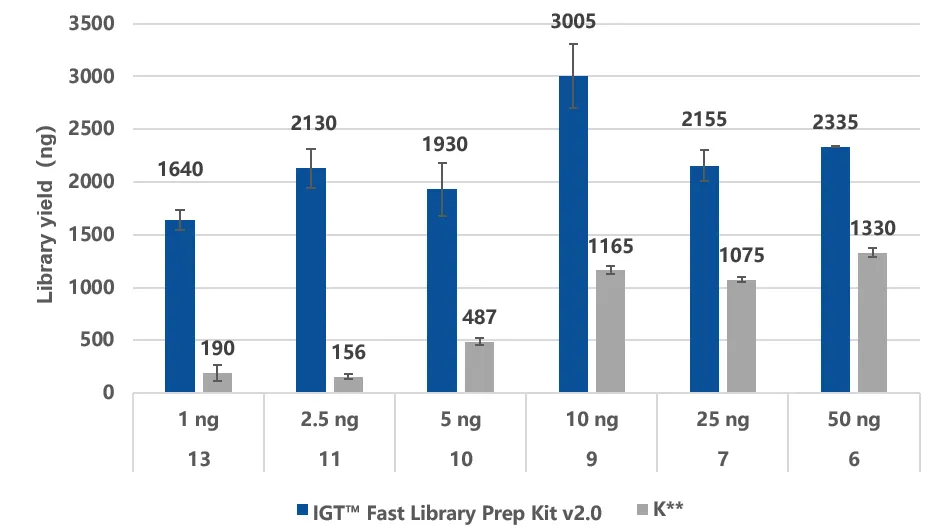
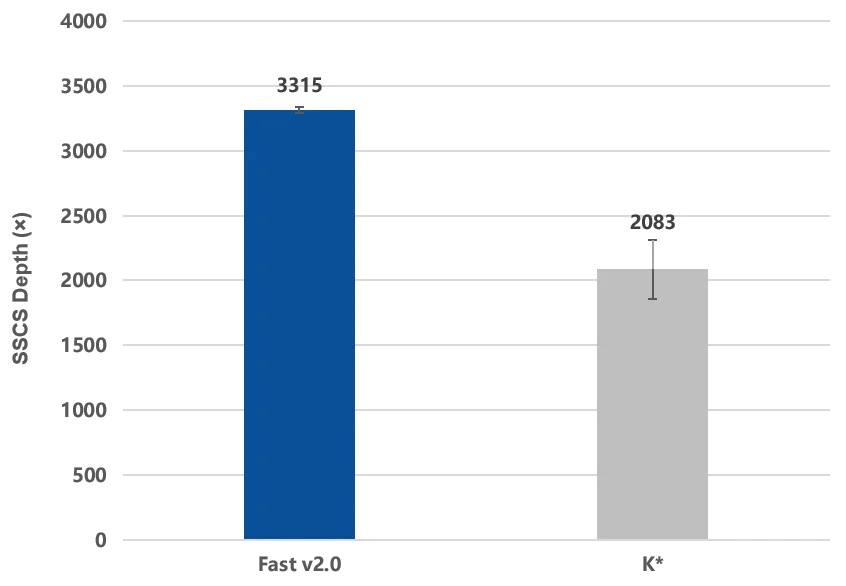
Figure 2. The IGT® Fast Library Prep Kit v2.0 enables efficient library preparation with a molecular recovery rate of over 50%.
A: Using simulated fragmented gDNA samples with input amounts of 1 ng, 2.5 ng, 5 ng, 10 ng, 25 ng, and 50 ng for library construction, with adapters as IGT™ UMI Adapter & UDI Primer (for Illumina), and amplification performed according to the recommended cycle number.
B: Using ctDNA standard samples with an input of 10 ng for library construction, and the captured library was sequenced on a NovaSeq 6000 PE150. After single-end UMI correction of the raw data, the depth of the target region was evaluated to calculate the molecular recovery rate.
In actual capture performance and variant detection tests, based on iGeneTech’s high-throughput MRD panel synthesis protocol and full-process supporting reagents, 7 MRD panels with different target sites synthesized in the same batch were randomly selected for capture performance testing. The results showed that stable and excellent capture performance was achieved across different panels and sites. Meanwhile, in the low-frequency mutation detection test, even at a mutation frequency as low as 0.01%, 5 mutation signals could still be stably detected among 8 mutation sites.
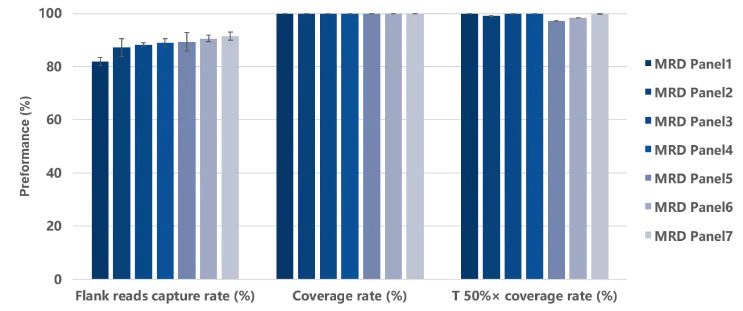
Figure 3. Data performance of capture testing using different MRD panels. Capture testing was performed using 7 MRD panels each containing 37 probes. The single hybridization library input amount was 750 ng, and sequencing was conducted on the NovaSeq 6000 platform with PE150 mode.
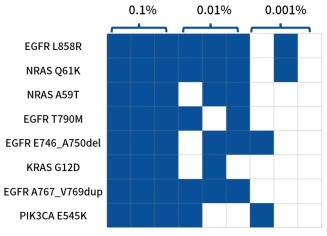
Figure 4. Detection statistics of known mutation sites in 8 positive reference materials at mutation frequencies of 0.001% to 0.1%. Library preparation was performed with 50 ng input of Horizon ctDNA reference materials (HD780) at mutation frequencies of 0.1%, 0.01%, and 0.001%. A random MRD panel containing 37 probes was used for target capture, and raw sequencing data were analyzed using iGeneTech®’s UMI-based data analysis pipeline. The number of detected mutations after single-end UMI correction was counted.
Increasing the number of individualized MRD monitoring sites allows for minimal blood collection, balancing the physical and psychological well-being of patients while ensuring the accuracy of MRD testing. To this end, iGeneTech offers a 150-probe MRD free customization service, combined with its in-house developed full-process high-molecular-recovery reagents. This approach enables smaller blood volume collection, lower testing costs, and the detection of lower-abundance ctDNA molecules throughout the testing process.
References
[1]. Https://meetings.asco.org/abstracts-presentations/203770/slides
[2]. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer[J]. Science translational medicine, 2015, 7(302): 302ra133-302ra133.3
[3]. Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer[J]. Science translational medicine, 2016, 8(346): 346ra92-346ra92.
[4]. Jeanne, Tie,Yuxuan, Wang,Cristian, Tomasetti et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer.[J] .Sci Transl Med, 2016, 8: 0.2.
[5]. Jacob J, Chabon,Emily G, Hamilton,David M, Kurtz et al. Integrating genomic features for non-invasive early lung cancer detection.[J] .Nature, 2020, 580: 0.3.
[6]. Alessandro, Leal,Nicole C T, van Grieken,Doreen N, Palsgrove et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer.[J] .Nat Commun, 2020, 11: 0.4.
[7]. Isaac, Garcia-Murillas,Neha, Chopra,Iñaki, Comino-Méndez et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer.[J] .JAMA Oncol, 2019, 5: 0.
[8]. Heitzer E , Haque I S , Roberts C , et al. Current and future perspectives of liquid biopsies in genomics-driven oncology[J]. Nature Reviews Genetics, 2018.
[9]. Knight, Kevin, Sally Wade, and Lodovico Balducci. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. The American journal of medicine 116.7 (2004): 11-26.
[10]. Deveson IW et al. Valuating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat Biotechnol. 2021 Sep;39(9):1115-1128. doi: 10.1038/s41587-021-00857-z.
[11]. Zviran A , Schulman R C , Shah M , et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring[J]. Nature medicine, 2020, 26(7):1-11.
[12]. Pol Y , Mouliere F . Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA[J]. Cancer Cell, 2019, 36(4):350-368.